Quality Management System
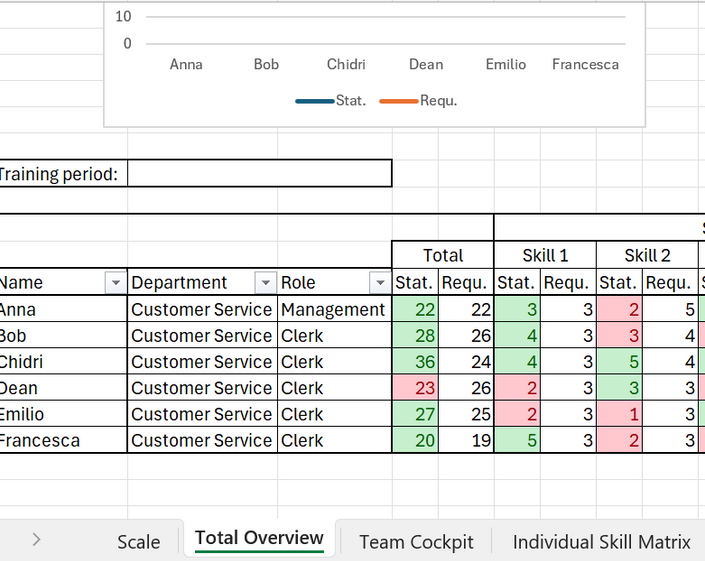
A strong Quality Management System (QMS) is vital in the pharmaceutical industry to ensure consistent product safety, efficacy, and regulatory compliance. Good Standard Operating Procedures (SOPs) are the backbone of a QMS, providing clear guidelines that help prevent errors and ensure high standards across all processes. A well-structured skill matrix ensures that every team member is qualified and proficient in their roles, directly impacting the quality of work. Effective audit planning further supports continuous improvement and compliance, identifying gaps and maintaining the integrity of pharmaceutical operations.
What You'll Gain:
This course provides a solid foundation in Quality Management Systems (QMS), including the essentials of SOPs, skill matrix development, audit planning, and SWOT analysis. With 13 engaging videos and 2 hours of in-depth content, you'll gain practical knowledge that you can immediately apply in the pharmaceutical industry. Active participation is key, as you'll be encouraged to dive deeper into the material through independent research and quizzes, helping you cement your understanding. By the end, you'll have a thorough grasp of QMS fundamentals and the tools to effectively implement them in your work.
Who It's For:
This course is ideal for individuals in the pharmaceutical sector with some work experience who are new to the quality aspects of their roles. It’s designed for professionals who are starting to engage with Quality Management Systems, SOPs, audits, and related processes.
Whether you're a newcomer to these topics or transitioning into a role that requires a deeper understanding of quality and compliance, this course will equip you with the essential knowledge to confidently handle quality management and audit preparation in the pharma industry.
Curriculum
Success is in your hands!
This course is designed to increase competency! Thus, it is more than watching videos.
The course includes active tasks for you. The more you are doing proactively, the more wins you will have.
Your Instructor
With a strong background in automotive customer support, Birte Demant-Keffel now serves as Compliance Officer and Drug Information Officer at ARC-Traicoa. Known for her exceptional attention to detail and commitment to quality, Birte ensures that all operations meet the highest ethical and legal standards. Her meticulous approach and dedication to regulatory compliance make her a key asset to the team, driving excellence in every aspect of her work.
She is glad to share her knowledge and insights into ISO standards and quality aspects.

Featured Products for Professionals
Level-up your career with further professional courses.